Why Functional Resolution Matters for Interpreting CTNNB1 Cancer Mutations
At Fulcrum, we’re often brought into projects where variants have already been called, annotated, and grouped. The harder question tends to come later: which of those differences actually matter for interpretation?
That question sits at the center of a newly published Nature Genetics paper co-authored by Fulcrum Principal Bioinformatics Scientist Alison Meynert, based on work completed prior to her joining Fulcrum. The study offers a concrete example of what becomes visible once functional differences between variants are measured directly, rather than inferred.
A closer look at a familiar hotspot
CTNNB1 exon 3 mutations are common across multiple cancer types and are typically described as activating β-catenin signalling. While that description is broadly correct, it glosses over meaningful variation within the hotspot.
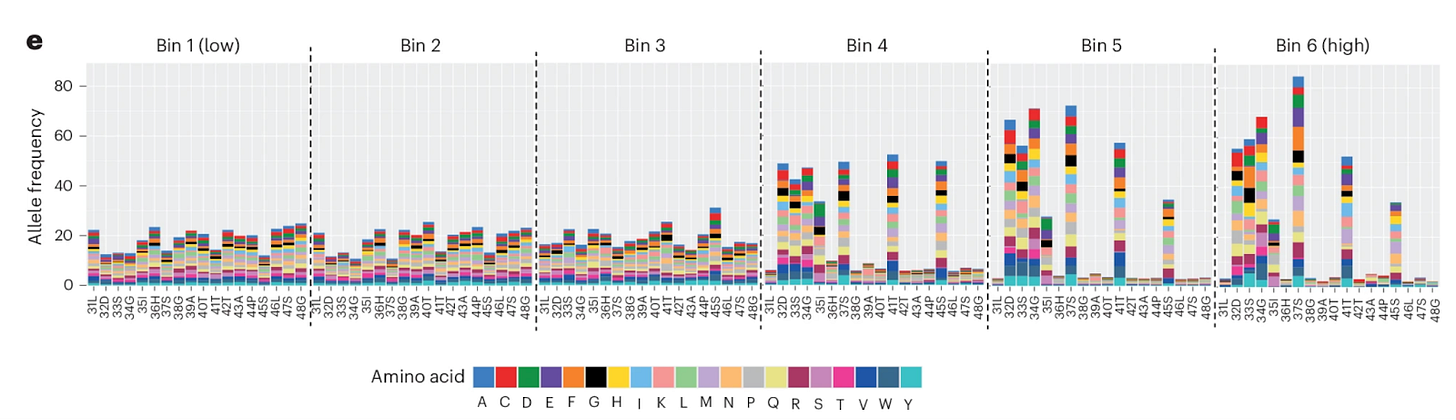
In this work, the authors used a saturation mutagenesis strategy to generate and measure all 342 possible missense mutations across the exon 3 region. By combining a β-catenin reporter assay with flow sorting and deep sequencing, they quantified signalling output for each variant under endogenous regulatory control.
The result is a functional spectrum rather than a single category.
What functional measurements add
One of the more striking observations is that mutation frequency does not explain functional impact.
The team calculated mutational likelihood scores based on background nucleotide substitution rates in hepatocellular and endometrial cancers, accounting for all single-nucleotide paths between codons. Those probabilities did not predict which mutations are observed in tumours.
Instead, different tissues preferentially accumulate mutations that fall within particular ranges of β-catenin activity.
Mutations that sit close together in sequence space can differ substantially in effect size. And treating them as interchangeable hides that structure.
Figure 1e from Krishna, et al. Mutational spectra changes with strength of B-catenin activity for each flow-selected pool.
When functional differences surface clinically
When hepatocellular carcinoma samples were stratified by measured signalling strength, rather than by mutation presence alone, clinically relevant patterns emerged.
Tumours with relatively weakly activating CTNNB1 mutations showed poorer survival than those with stronger activating mutations. These same tumours also showed greater immune cell infiltration.
One interpretation, proposed by the authors, is that tumours may select for a “sweet spot” of β-catenin activation within the WNT pathway that’s strong enough to support growth, but not so strong that it triggers counterproductive immune or regulatory responses. That optimal range appears to vary by tissue type and may interact with other dysregulated pathways.
From the tumour’s perspective, not all pathway activation is equally advantageous.
Without functional stratification, these associations are not apparent.
Carrying this perspective forward
Although the work for this study predates Alison’s time at Fulcrum, the perspective it reflects is familiar in her work today: a reluctance to overgeneralize and a focus on resolving heterogeneity before drawing conclusions.
For clients, that often shows up as how questions are framed early. We ask what assumptions are worth challenging and where additional resolution is likely to change interpretation rather than add noise.
📖Read the paper:
Mutational scanning reveals oncogenic CTNNB1 mutations have diverse effects on signalling and clinical traits